Peripheral T-cell lymphoma (PTCL) represents a heterogenous group of aggressive non-Hodgkin lymphomas with poor prognostic outcomes and limited treatment options. Despite the advances in genomic sequencing and molecular characterization, the complex and elusive nature of PTCL's underlying biology has proven difficult to fully understand and manage effectively. The development and refinement of therapeutic strategies for PTCL are impeded by a paucity of reliable preclinical models that accurately mimic the disease's pathophysiology. There is a dire need for more physiologically relevant models for PTCL. Improved models allow for a better understanding of the disease's molecular and cellular mechanisms, facilitate the development of innovative therapeutic strategies, and ultimately improve patient outcomes.
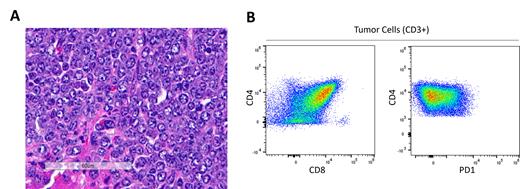
Here we described a new T-cell lymphoma cell line (LM-23) derived from a 12-week-old female Balb/cJ mouse which developed diffuse adenopathy and splenomegaly. Tumors from this mouse were cultured in cRPMI + 10% fetal bovine serum for 4 weeks without supplementation of additional cytokines and passaged 3-4 times per week where we found the cells to be non-adherent. Immunophenotyping of LM-23 demonstrated positivity for CD90, CD3, TCR-beta, CD4(partial/subset), CD8, PD1 (positive), ICOS (positive) and negative for B-cell and myeloid lineage markers (Fig. 1B). This immunophenotype most closely resembles PTCL-NOS in humans at an immunophenotypic characterization level. Whole exome sequencing is in progress and will be presented.
Intravenous administration of this cell line to syngeneic Balb/cJ mice resulted in rapid establishment of diffuse adenopathy, splenomegaly, and liver metastases. Bulky adenopathy was observable within 14 days of administration. Histologically, these tumors consisted of sheets of medium to large sized lymphocytes with clumped chromatin and prominent nucleoli, similar to human T-cell lymphomas (Fig. 1A). Subcutaneous administration demonstrated rapid tumor growth with mice reaching terminal endpoints (>2,000mm 3) within 21 days following administration. As CHOP-based (cyclophosphamide, doxorubicin, vincristine, and prednisone) therapies are the primary backbone for treatment of PTCL, we tested whether CHOP therapy alone had anti-tumor activity to LM-23. Tumor bearing mice (~150mm 3) were treated with CHOP and monitored for tumor growth. All mice treated were non-responsive to CHOP therapy with no difference in tumor growth compared to untreated mice. Additionally, LM-23 bearing mice treated with anti-PD1 showed signs of hyperprogression, which has been observed in human patients in clinical trials (Gao Y et al. JCI Insight 2023, Bennani NN et al. Blood Supplement 2019). Additional therapeutic target testing is underway and will be presented.
In conclusion, the newly established LM-23 T-cell lymphoma cell line provides a novel, physiologically relevant preclinical model for the study of PTCL. Its phenotypic characteristics, rapid growth, and metastatic behavior in syngeneic mice, along with its resistance to CHOP therapy and hyperprogression upon anti-PD1 treatment, mimicking the clinical scenarios observed in some human PTCL patients. These features highlight LM-23's potential as a valuable tool for understanding the biological mechanisms underlying PTCL and for the development and testing of innovative therapeutic strategies. We hope that the LM-23 cell line will contribute significantly to these efforts, ultimately improving outcomes for patients afflicted with PTCL.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal